Abstract
Background: CT053 is a fully human autologous chimeric antigen receptor (CAR) T-cell therapy comprising a B-cell maturation antigen (BCMA)-specific single-chain variable fragment (25C2). A clinical trial of CT053 is ongoing in LUMMICAR STUDY 1 (NCT03975907) for patients with relapsed and refractory multiple myeloma (RRMM) in China. The pivotal Phase 2 of LUMMICAR STUDY 1 is actively enrolling patients. Here, we report clinical data from Phase 1 with 12 months of follow-up.
Methods: Subjects with RRMM who had received ≥3 prior therapies, including at least one proteasome inhibitor and one immunomodulatory drug were enrolled in Phase 1 study. Adverse events (AEs) were graded according to CTCAE, v5.0; Cytokine release syndrome (CRS) and neurotoxicity were graded according to ASTCT CRS consensus grading system (Lee DW et al, 2019). Response was assessed per IMWG 2016 criteria. Minimal residual disease (MRD) was tested by next-generation flow cytometry on bone marrow aspirates by the EuroFlow assay with a minimum sensitivity of 1 in 10⁵ nucleated cells or higher, and CAR copies in the subject peripheral blood were monitored by quantitative real-time polymerase chain reaction (qPCR).
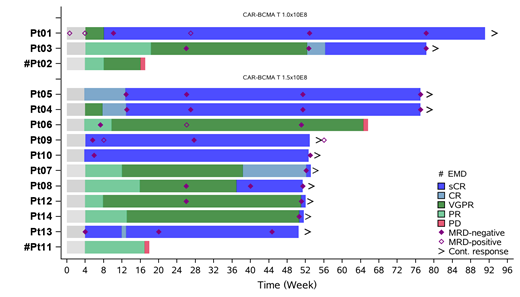
Results: Fourteen subjects with a median age of 54 years (range 34-62) received CT053 infusion. Three subjects received 1.0×10 8 CAR+ T cells and eleven subjects received 1.5×10 8 CAR+ T cells. As of July 8, 2021, 14 subjects with a median follow-up of 13.6 months since infusion. Of the 14 subjects, 11 (78.6%) had received autologous stem cell transplantation, 2 (14.2%) had extramedullary disease at baseline, 2 (14.3%) had ISS stage III, and 5 (35.7%) had high-risk cytogenetics. The median prior therapies was 6 (range 3-7) and no subject received bridging therapy.
The most common AEs were expected hematological toxicities. All subjects (100%) experienced ≥ grade 3 neutropenia, 91.7% experienced ≥ grade 3 thrombocytopenia, and most recovered to ≤ grade 2 within 2 weeks. No dose limiting toxicity or treatment-related death was reported. Additionally, no ≥ grade 3 CRS or neurotoxicity was observed, 92.9% subjects (13/14) experienced grade 1 or 2 CRS (9 grade 1, 4 grade 2). CRS occurred at a median of 6 days (range 2-12) post-infusion with a median duration of 7 days. No severe infections were reported except one case of grade 3 lung infection.
A 100% overall response rate was achieved in 14 subjects, with 11 stringent complete responses (sCR, 78.6%), 2 very good partial responses (VGPR), and 1 partial response, with a ≥VGPR rate of 92.9%. Four subjects achieved sCR at week 52. As of July 8, 2021, 12 subjects with at least 12 months of efficacy assessment were still in the study, and the 12-month progression-free survival (PFS) rate was 85.7%. With a median follow-up of 13.6 months, the median duration of response and the median PFS had not been reached. All 11 subjects with sCR were MRD-negative, and 9 subjects reached sustained CR/sCR for more than 12 months (Figure 1). Three subjects had disease progressed including two subjects with extramedullary disease progressed at week 16. Interestingly, the CR/sCR rate for the subjects without extramedullary disease is 91.7% (11/12). The 1-year PFS rate for subjects without extramedullary disease reached 100%。
CT053 cells expanded and persisted well. No immunogenicity was detected.
Conclusion: These results demonstrate that CT053 CAR T cells at a dose of 1.0-1.5×10 8 cells achieve a deep and durable response, including a high MRD-negative sCR rate, with an acceptable safety profile in subjects with heavily pretreated RRMM.
Li: CARsgen: Current Employment, Current equity holder in publicly-traded company. Wang: CARsgen Therapeutics Corp: Current Employment. Xiao: CARsgen Therapeutics Corp: Current Employment. Wang: CARsgen Therapeutics Co. LtD: Current Employment, Current equity holder in publicly-traded company. Tang: CARsgen Therapeutics Corp: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal